CLEMSON, South Carolina — New research stemming from scientists at Clemson University has optimized a novel imaging technique that could help doctors better visualize tumors.
The research – published recently in the International Journal of Nanomedicine – expanded upon a technique known as multi-photon imaging, which uses near-infrared light to excite fluorescent molecules that are injected near the site of a tumor. The multi-photon approach enables light to penetrate deeper into tissue, capturing even the smallest of tumors that would otherwise go undetected by traditional imaging techniques, such as an MRI or PET scan.
Once tumor cells are injected with the fluorescent molecules, typically two photons, or particles of light, are then aimed at the tumor cells, exciting the electrons within the fluorescent molecules. As the electrons relax back to a lower energy state, the molecules emit fluorescent colors of red, green or blue, which are pictured by an imaging device. The wavelengths of the two exciting photons are longer than the wavelength of the emitted fluorescence, explaining how multi-photon imaging can manage to penetrate several millimeters deep into tissue.

“What we have done in this study is three-photon absorption, which can penetrate even deeper than two photons. The problem, however, is that we need very high power to excite three photons, but higher power could cause fluorescent molecules to lose their fluorescent ability or become toxic to patients,” said Achyut Raghavendra, a graduate student in the department of physics and astronomy and the lead author of the study.
To combat these limitations, researchers have recently began testing nanomaterials in the development of fluorescent dyes for multi-photon imaging; although, many of the proposed materials have fallen short. Cadmium selenide, for example, could perform under longer wavelengths of light, but became toxic in doing so. Nanostructures made of gold and silver were also a contender, but they were found to scatter light and become toxic when tested.
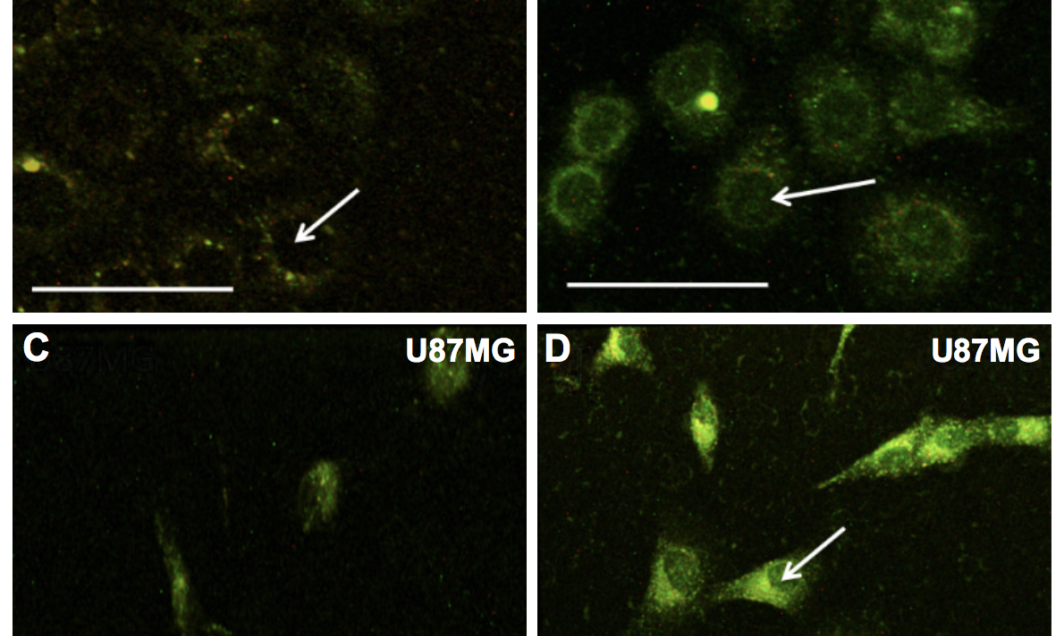
The team instead focused on zinc oxide, a semiconducting nanoparticle that is characterized by its luminescence and its ability to perform well with little microscopic power.
“We outfitted the zinc oxide nanoparticles with some specific functional groups so that they could selectively attach to one type of cancer cells and not the healthy cells surrounding the tumor,” said Ramakrishna Podila, a professor in the department of physics and astronomy and a co-author on the study.
By functionalizing zinc oxide with a group of amino acids – arginine, glycine and aspartic acid – the nanoparticles were directed to target brain and breast cancer cells. Once attached, the functional group acted as a sort of “key,” unlocking access to the inside of the tumor cells and allowing the nanoparticles to flow in.
The nanoparticles were also purposely defected by the researchers to maximize their fluorescence, creating the other point of novelty in the study.
“The main defects are missing oxygen atoms or extra zinc atoms,” Podila said. “Zinc oxide is ideally supposed to be one part zinc and one part oxygen, but we are making it 1.1 parts zinc and 0.9 parts oxygen, for instance. The green color emission is coming out because of these defects.”
Moreover, biocompatibility studies with zinc oxide found that the molecule did not cause any damage to human cells when excited by light, suggesting the nanoparticle is safe for use in this application.
Combined, the findings reveal a technique for cancer imaging that is customizable for a variety of cancer types and can lead to more successful tumor resections by helping doctors visualize the boundaries of a tumor mass.
Terri Bruce, director of the Clemson Light Imaging Facility and a coauthor on the study, spoke of the importance of this point.
“In order to navigate a successful tumor resection, a neurosurgeon must be able to delineate tumor cells from healthy tissue in order to ensure the most successful outcomes – a successful outcome being as much tumor removal as possible without disruption of healthy brain tissue,” Bruce said. “This new technology represents a crucial step in the development of more sensitive imaging techniques for use in a variety of cancers.”
Though there are currently no clinically available multi-photon instruments, Podila said that this is a developing field of study that is sure to see improvements in the future.
END
The team’s publication, titled “Three-photon imaging using defect-induced photoluminescence in biocompatible ZnO nanoparticles,” was supported by an R-01 grant from the National Institutes of Health National Institute of Environmental Health Sciences under grant number ES019311-07, as well as a National Science Foundation MRI award (#1126407). The researchers are wholly responsible for the content of this study, of which the funder had no input.
Get in touch and we will connect you with the author or another expert.
Or email us at news@clemson.edu